General
FDA says some Pfizer vaccine vials contain extra doses
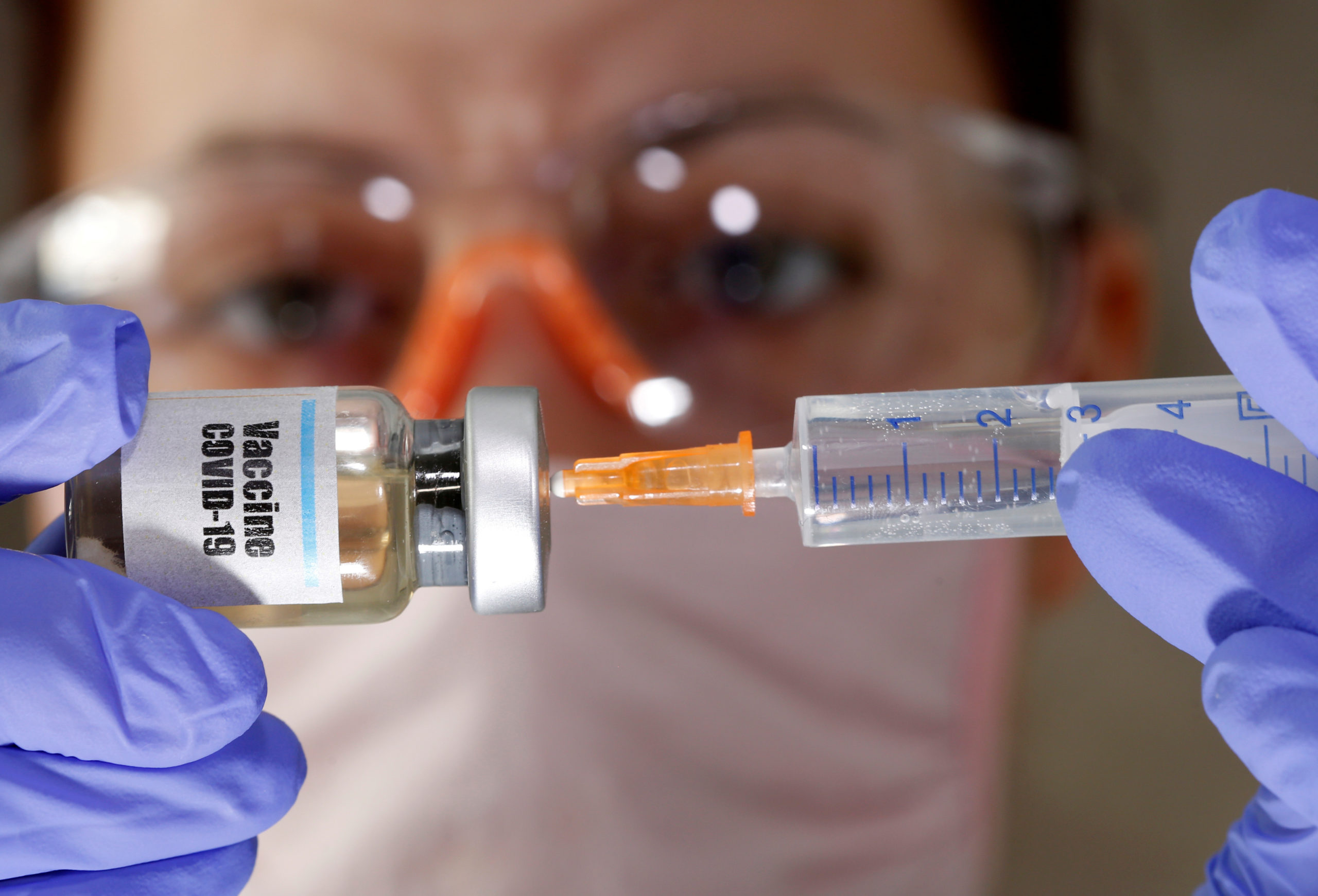
The U.S. Food and Drug Administration said Wednesday that some vials of the Pfizer/BioNtech vaccine contain extra doses apparently boosting the U.S. supply of vaccines.
In a statement on Twitter, the FDA said some vials that are supposed to hold five doses possibly contain six or seven doses.
“At this time, given the public health emergency, FDA is advising that it is acceptable to use every full dose obtainable (the sixth, or possibly even a seventh) from each vial, pending resolution of the issue,’’ the statement said.
The FDA added that it is working with Pfizer to determine the “best path forward”.
The announcement means the U.S. could see a significant increase in its vaccine supply, which would be a welcome development for the country which is scrambling to obtain more doses.
The U.S. has purchased 100 million doses of the Pfizer vaccine – enough to inoculate 50 million people – but most of those doses will not be available for months.
The U.S. has also purchased 100 million doses of the Moderna vaccine, which was found to be highly effective in a trial but has yet to receive approval from the FDA.
Meanwhile, a health care worker in the U.S. state of Alaska was hospitalised over what is presumed to be an adverse reaction to the Pfizer/BioNtech COVID-19 vaccine, the local health authority said Wednesday.
The worker is in stable condition but remains in hospital for monitoring after she suffered an anaphylactic reaction that included shortness of breath 10 minutes after receiving the vaccine dose on Tuesday.
The person had no history of allergies, the Alaska Department of Health and Social Services said in a statement.
The health worker’s allergic reaction comes after two health workers in Britain also suffered from adverse reactions leading the British medical authority to advise against administering the shot to people with a history of severe allergic reactions.
“We expected that a side effect like this could occur after reports of anaphylaxis were made in England after people there received the Pfizer-BioNTech COVID-19 vaccine,’’ Alaska’s Chief Medical Officer, Anne Zink, said in a statement.
“All sites that are approved to provide vaccinations in Alaska must have medications on hand to deal with an allergic reaction and that was the case in Juneau,’’ she added.
The first doses of the vaccine were administered in the U.S. on Monday and continuing through Wednesday with a focus on front-line health care workers.
NAN
-

 News22 hours ago
News22 hours agoKenneth Okonkwo knocks CBN’s forex management amid economic struggles
-

 News22 hours ago
News22 hours agoNavy nabs 14 criminals in Akwa Ibom
-

 News17 hours ago
News17 hours agoGoldman Sachs donates $5000 to Tunde Onakoya’s world record fundraiser
-

 News21 hours ago
News21 hours agoUTME: JAMB calls for calm amid technical glitches
-

 Entertainment18 hours ago
Entertainment18 hours agoBurna Boy, Nollywood receive praise from Canadian senate
-

 Education18 hours ago
Education18 hours agoPlateau varsity mourns student’s death, suspends exams
-

 News7 hours ago
News7 hours agoGWR: Tunde Onakoya set a new 58-hour record, hits 60 hours
-

 News7 hours ago
News7 hours agoPolice arrest Yahaya Bello’s ADC, others



































